INTRODUCTION TO PREPARATIVE SFC
Purification is to free from anything that debases, pollutes, adulterates, or contaminates.
First Step in chemistry, it is the separation of a substance into its components and the removal of impurities.
Since ancient times, people have used methods of separating and purifying chemical substances for improving the quality of life.
In everything from pharmaceutical discovery, to chemical materials, natural products and food production, purification is a necessity.
The goals of purification in general are to isolate a substance, alter the composition of a mixture, or remove interferences.² Specifically, the reasons for purification are broad-ranging; structural elucidation, activity studies, product formulation and enrichment, impurity profiling, and many more.
There are many solutions of varying complexity and effectiveness to meet the challenge of purification. However, purification and isolation of compounds in a high-throughput environment is often a bottleneck to productivity.
This makes faster and more efficient tools a critical need.³ Supercritical fluid based technology (SFC) encompasses a variety of CO2 based techniques designed to streamline and simplify the steps involved in a purification workflow.
One of these technologies is preparative supercritical fluid chromatography (Prep SFC).
In this primer, the principles, application, instrumentation, and workflow of Prep SFC as a purification technology will be introduced.
CO2 AS A SOLVENT
A supercritical fluid results when a fluid is above its critical pressure and critical temperature.
At the critical point, the interface between liquid and gas phases disappears and a highly compressed gas results with densities similar to liquid densities.
Supercritical fluids also exhibit high diffusivity and low viscosity similar to a gas.
The solvating power of a supercritical fluid is mainly related to density, which can be tuned by manipulating pressure and temperature.
Generally, a higher density implies stronger solvating capacity.⁴ Increased density results from either a decrease in temperature or an increase in pressure.
On the other hand, a decrease in density is observed with an increase in temperature or a decrease in pressure.⁵ Figure 1 shows the phase diagram for CO2; the physical changes from one state to another and the critical point are indicated.
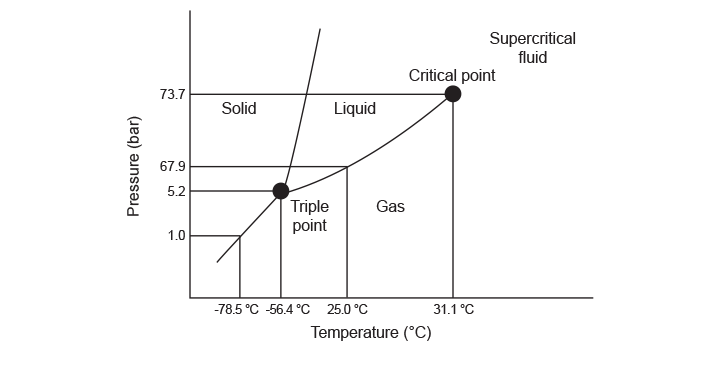
Many substances require extreme conditions to reach their supercritical state, and exhibit undesirable properties when in that state. In Table 1, the supercritical fluid conditions and accompanying properties of some selected supercritical substances are shown. Unlike other supercritical substances, CO2 is generally regarded as safe because it is not flammable, explosive, toxic or corrosive.³ Because the supercritical state of CO2 is easily achievable, at 31°C and 74 bar, the density can be manipulated at temperatures and pressures within an acceptable range.⁵ Also, due to the relatively mild critical temperature, it is amenable towards thermally labile samples. CO2 is also relatively inexpensive because it is easily recovered from other industrial processes; this means it has a neutral impact on environmental CO2 levels.⁶ All of these advantages make CO2 the most common substance utilized in supercritical fluid technologies.
| Substance | Critical temp. (ºC) | Critical pressure (bar) | Comments |
| Carbon dioxide | 31 | 74 | Physical state easily changed |
| Water | 374 | 221 | Extreme conditions needed |
| Methanol | 240 | 80 | Extreme temperature needed |
| Ammonia | 132 | 111 | Highly corrosive |
| Freon | 96 | 49 | Environmentally unfriendly |
| Nitrous Oxide | 37 | 73 | Oxidizing agent |
| n-Butane | 152 | 38 | Highly flammable |
Table 1. Critical temperatures and pressures for selected substances, and notable properties of those substances.
PURIFICATION USING AN SFC WORKFLOW
A purification workflow consists of multiple steps, ranging in complexity and necessity based on the requirements of the application. At a basic level, an SFC purification workflow contains the following components
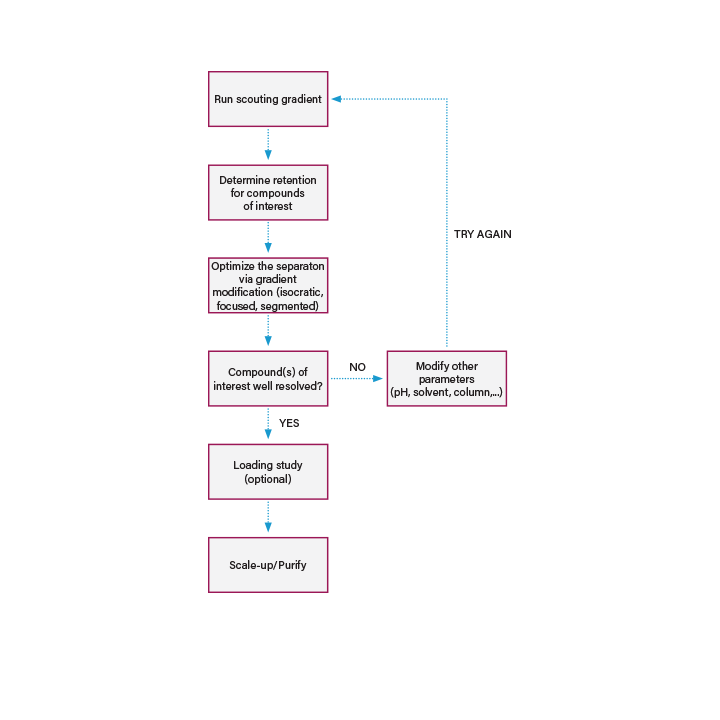
(Figure 2):
Starting Material or Sample: The sample can be complex, as in a naturally occurring botanical, or relatively simple, like a well characterized pharmaceutical candidate. This will determine how much, if any, sample preparation is required, and the scale of purification. It is also good to have as much information as possible about the sample and eventual product, such as thermal stability, polarity, solubility and reactivity which dictates how that sample can be treated.
Sample Preparation: In the first step of the purification process, the sample needs to be prepared appropriately depending on the state of the starting material and the goal or scope of the application. Sample preparation can have many steps such as grinding, drying, extracting and filtering, or simply dissolving the sample in solution. Supercritical fluid extraction (SFE) is the first (sample preparation) step in an SFC workflow. It is typically used in applications involving raw industrial products, biobotanicals, or natural products.
Sample Purification: Purification is used to decrease sample complexity or isolate a final product to within a certain purity spec for analysis or product formulation. Samples prepared by SFE are typically complex mixtures containing target compounds and impurities. In an SFC workflow, preparative supercritical fluid chromatography (Prep SFC) is the second step (purification), where one or multiple targets are purified from an extract. Prep SFC can also be applied to samples prepared using many different methods, not just by SFE.
End Product: The end product is the final goal of the workflow. It can be data and information generated by analysis, a refined material used in a process, or a final product that can be utilized directly. The end product dictates the instrumentation and methodology required for a successful workflow. In an SFC workflow, analysis of samples pre and post extraction (SFE) and purification (Prep SFC) are performed using Ultra Performance Convergence Chromatography (UPC2).
Any of these SFC technologies can be utilized, as required, in non-SFC workflows, as preparation, purification, or analysis steps.
INTRODUCTION TO PREP SFC
PREPARATIVE CHROMATOGRAPHY: PROGRESSION FROM HPLC TO SFC
Preparative high-performance liquid chromatography (Prep HPLC) has been one of the most frequently used techniques for purification for over 20 years.⁶ Specifically, it is a popular separation process in the fine chemicals, pharmaceutical and biotechnology industries where it is widely used for the purification of products.⁵ Over this time, Prep HPLC has developed into a very efficient and applicable technique, particularly for achiral purification. Reversed-phase liquid chromatography (RPLC) has the advantage of using a quasi-universal stationary phase (C18) and a generally applicable mobile phase mixture of water and acetonitrile. RPLC is compatible with mass spectrometry (MS), and RPLC paired with MS (RPLC-MS) has been the standard approach for purification in many research environments. ⁷
Despite its popularity, Prep HPLC has several drawbacks. The volume of mobile phase needed to purify a given mass of compound is large compared to the amount of overall sample that is processed. Typical Prep HPLC fractions contain large volumes of solvent (both organic and aqueous) which creates a bottleneck to productivity because of the time and energy required to dry down and procure the final product.
The solvents used in LC can pollute the environment, both locally (through evaporation and exposure) and at large through the burning of the chemical waste. Normal-phase liquid chromatography (NPLC) is considered even more environmentally harmful, as the mobile phase is typically comprised of 100% organic solvent. Because of these environmental factors, the procurement and disposal of solvents used in LC is becoming increasingly costly; creating an incentive for more solvent-free or greener processes.⁵ SFC is an alternative technique, which can help alleviate the bottleneck and bring about operational refinements that shorten timelines, reduce solvent waste and cut costs.⁸ Recently, progress in SFC instrumentation has led to renewed interest in the technique as a powerful tool for chiral and achiral purification.⁷ SFC is an environmentally “greener” alternative to HPLC for analysis and purification.6,3
SFC IS CHROMATOGRAPHY!
Supercritical fluid chromatography (SFC) is a chromatographic technique that uses sub-critical (liquid) and supercritical CO2 as the primary solvent in the mobile phase, usually accompanied by an organic solvent. Like all chromatography, SFC separates components based upon the partitioning of analytes between a stationary phase (column) and a mobile phase (solvent).⁵ There are many similarities between HPLC and SFC, for example: SFC can be run using both isocratic and gradient method conditions and is compatible with all standard detection techniques such as ultraviolet (UV), photodiode array (PDA), evaporative light scattering (ELS) and mass spectrometry (MS). The general Prep SFC workflow is the same as for HPLC involving method development, scale-up, fraction collection and purity analysis of the collected fractions (Figure 3). It is also comparable to RPLC in terms of recovery and purity, for some applications recovery is better in SFC while for others HPLC is the better solution. ⁷
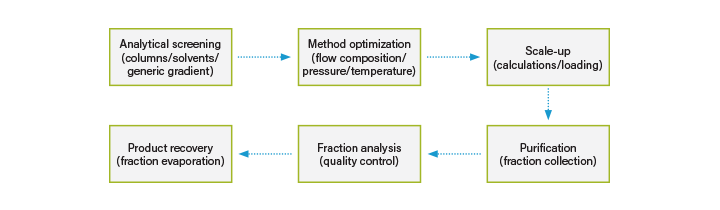
Figure 3. Preparative chromatography workflow.
SFC generally leverages the principles of normal-phase chromatography. What makes SFC different than HPLC, is the use of CO2 as the primary component of the mobile phase⁵; replacing non-polar liquid components, such as hexanes and heptanes. Because supercritical CO2 is a compressible fluid, pressure and temperature become important parameters used to control solvent strength, impacting retention and selectivity.7,9 Supercritical CO2 is favorable for chromatographic purification because it is non-flammable, non-toxic, and has high diffusivity, low viscosity and excellent solvating power.10 SFC has become attractive to purification labs in recent years, by providing significant advantages in terms of solvent savings and productivity.
PREP SFC ADVANTAGES: SOLVENT SAVINGS
A major advantage of Prep SFC is lower solvent usage achieved by replacing a majority of the mobile phase with CO2.⁶ At the analytical scale this advantage may be small, but on the preparative scale the advantage is quite significant. In many purification labs, a significant amount of time can be spent on solvent removal from collected fractions, creating a bottleneck between purifying a compound and obtaining the desired target product or outcome. In Prep SFC, the CO2 portion of the mobile phase is removed at depressurization, leaving only a small amount of co-solvent. The resulting fractions have higher product concentrations, which reduces the time required for solvent removal and product isolation.⁶ The fraction can also be directly analyzed without a need for sample enrichment or concentration steps. This is of particular importance for compounds that decompose quickly under the normal, lengthy, dry down conditions.³
Other advantages of the lower organic solvent usage in SFC are cost savings, safety with respect to flammability and toxicity, and reduced impact on the environment. There is a considerable cost advantage in terms of procurement and disposal of solvent; however there are also savings due to the lower energy consumption needed for solvent removal.⁷ SFC can also avoid the use of toxic solvents such as acetonitrile used in RPLC, and the aliphatic hydrocarbons and chlorinated solvents used in NPLC. CO2, as a solvent, is relatively inexpensive because it is a byproduct coming from other industrial processes and it is recyclable.
PREP SFC ADVANTAGES: INCREASED PRODUCTIVITY
In SFC, productivity is increased due to the low viscosity and high diffusivity of the mobile phase, which improves chromatographic speed and efficiency.³ Figure 4 shows a comparison of the Van Deemter curves of HPLC, UPLC®, SFC, and UPC². In chromatography, the speed of separations is dictated in part by how fast the solute diffuses in the mobile phase,11 and into and out of the stationary phase. The Van Deemter curves for SFC are broader and flatter than for HPLC indicating that the efficiency of the chromatography remains high (low plate height values) as the flow rate (linear velocity) increases. In SFC, the higher diffusion coefficients translate directly to higher speed chromatography.
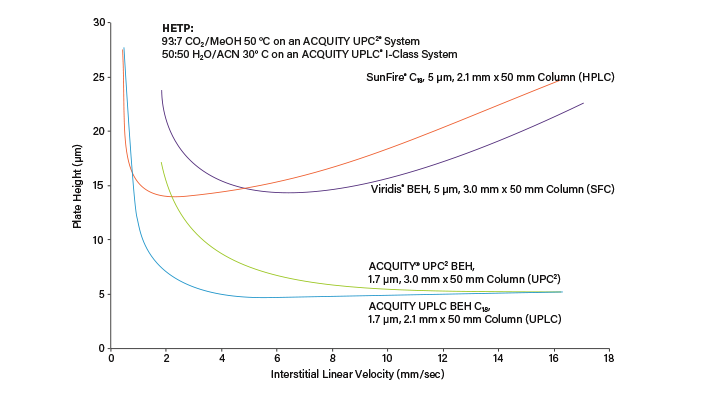
Due to the lower viscosity of the mobile phase, column and system pressures are lower, allowing for linear velocities up to 3 to 4x those seen in HPLC, and the use of smaller particle size columns.6,7 The low viscosity also results in shortened equilibration times. The result is short run times with high separation efficiency, leading to increased loading capacity and a fast injection cycle time; key parameters for improving productivity in any preparative chromatography process. Pure compounds can, therefore, be generated in a shorter time frame, resulting in higher overall productivity.6,7 An example of the time and productivity savings between HPLC and SFC can be seen
in Table 2.³
| Purification by SFC | Purification by HPLC | |
| Separation Time | 3 hours | 46 hours |
| Organic Solvent Used | 5 L of Methanol | 40 L of Acetonitrile |
| Total Workup Time | 1 hour | 8 hours |
| Recovery | 95% | 80% |
Table 2.
Comparison of preparative SFC vs. preparative HPLC (example data provided by Merck & Co.).³
PREP SFC IS ORTHOGONAL TO REVERSED-PHASE LIQUID CHROMATOGRAPHY (RPLC)
The orthogonal relationship between SFC and RPLC provides an opportunity to improve product quality in many applications. RPLC applies the C18 column as a nearly universal solution, which greatly simplifies method development, but the aqueous mobile phase limits the range of solvent compatibility and compound solubility. SFC, on the other hand, works well with a wide range of organic diluents resulting in a wider range of compatibility with solvents and compounds. It also has a wide range of stationery phases to choose from.
SFC is orthogonal to RPLC because it is generally regarded as normal phase chromatography and provides a diversity of separation options in an easy to use chromatographic format. In particular, by pairing normal phase selectivity with high separation efficiency, SFC has an advantage when separating stereo isomers, positional isomers, and structurally similar compounds.⁵ For non-polar compounds, the flexibility of SFC allows for use of reverse-phase columns (such as C18), while adding water as an additive extends the application range into the more polar region.10 In those applications where the compounds are easily degraded, SFC purification is an ideal alternative because separations are performed quickly, without water, and fraction dry down is completed at low temperatures and in less time.
Prep SFC is comparable to RPLC in terms of recovery and purity, and there is an overlap in applications that can be done by both SFC and RPLC. Figure 5 shows the results of a study in which a pharmaceutical library of compounds was screened for purification by SFC and LC. Approximately 82% of the compounds could be purified by either technique.12 However, the study also shows how the two platforms are complimentary. Some compounds could only be purified by SFC (4%) while others could only be purified by LC (8%). The flexibility afforded by pairing the two platforms provides a greater opportunity for optimizing the separation and purification. SFC provides a selectivity that is complimentary to RPLC, allowing for an orthogonal approach in method development and separation of challenging complex samples.⁸ By performing multi-step purification across platforms, or using an orthogonal technique for fraction analysis, a purer product can be recovered and more information obtained. An example of purification of a compound from a complex matrix using orthogonal LC and SFC separations is displayed in Figure 6.
Preparative SFC and LC Sample Screening
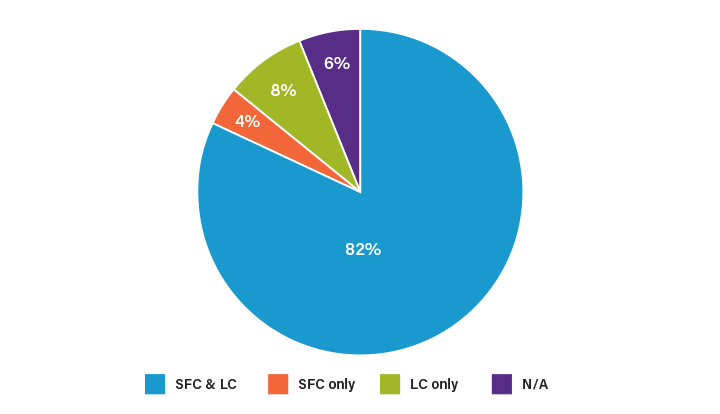
Figure 5. Chart showing the sample purification success rate for compounds screened using SFC and LC.12
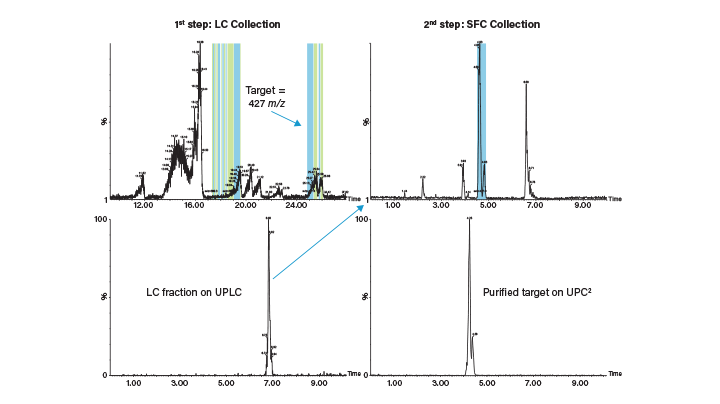
Figure 6. Example of a target compound purification from a natural product extract using two-step orthogonal purification by LC (1st step) and SFC (2nd step) .
